Role of Vagal Signaling in Feeding
The vagus nerve is a key part of the nutrient sensing machinery that controls meal termination and food choice.1 In diet-induced obesity vagally-mediated gut-brain signaling is blunted resulting in increased food intake.2
We’re seeking to define the timing, extent, and mechanisms by which vagal sensory signaling fails during the development of obesity and to identify novel strategies to combat this disease

Microbiota-Driven Vagal Remodeling in Obesity
In collaboration with Claire de la Serre, PhD at Colorado State University
The gut hosts an ecosystem of bacteria, viruses and fungi that provide important benefits to metabolic and immune health.3,4 In obesity the composition of the bacteria changes, and the obese microbiota are necessary and sufficient for the development of obesity.5-7
We’re examining the hypothesis that alteration in the gut microbiota during obesity, promotes a pro-inflammatory environment causing vagal sensory neurons to reorganize their terminals impacting how the brain integrates information about a meal.

Gut-Brain Signaling and Hypertension
In collaboration with Jasenka Zubcevic, PhD at the University of Toledo
Hypertension, a higher than normal blood pressure, affects over a quarter of the US population and is a major risk factor of deadly cardiovascular disease. Despite the availability of anti-hypertensive medications only 25% of patients have their hypertension under control.8
Vagal populations that innervate the carotid and aortic arch sense changes in blood pressure, and relay this information to the brain to maintain a healthy blood pressure. A failure in this heart-brain signaling can account for hypertension. However, hypertension is also associated with an altered gut microbiota signature.9 We are testing the innovative hypothesis that a vagal gut-brain pathway is involved in the regulation of blood pressure.

Brain-Gut-Retinal Axis in Diabetic Retinopathy
In collaboration with Maria Grant, MD at the University of Alabama at Birmingham and Jason Frazier, PhD at the University of Florida
Diabetic retinopathy is the leading cause of blindness in US adults. All diabetic patients can develop retinopathy but the mechanisms remain unclear.10 We’re exploring the potential role of a novel brain-gut-retina pathway in the development of diabetic retinopathy
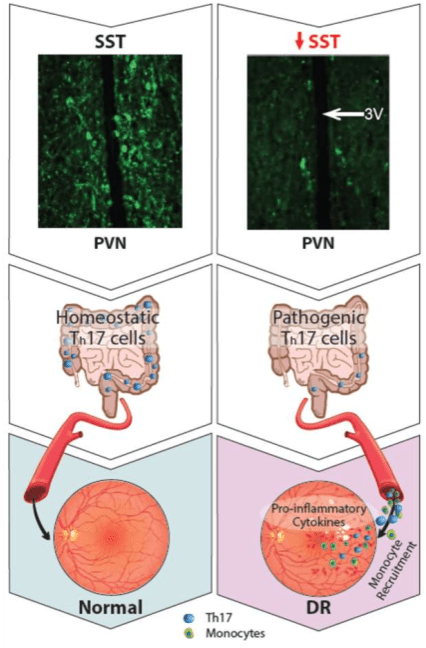
We hypothesize that, in diabetes, impairment of somatostatin neurons in the hypothalamus drives increased sympathetic nerve activity in the gut, which in turn promotes the transformation of a subset of T cells – Th17 cells – from a homeostatic to a pathogenic state. These pathogenic Th17 cells migrate from the gut to the retina where they participate in the retinal recruitment of innate immune cells, exacerbating diabetic retinopathy.

Vagal Oxytocin Receptors and Cardiometabolic Interoception
In collaboration with Eric Krause, PhD and Annette de Kloet, PhD at Georgia State University
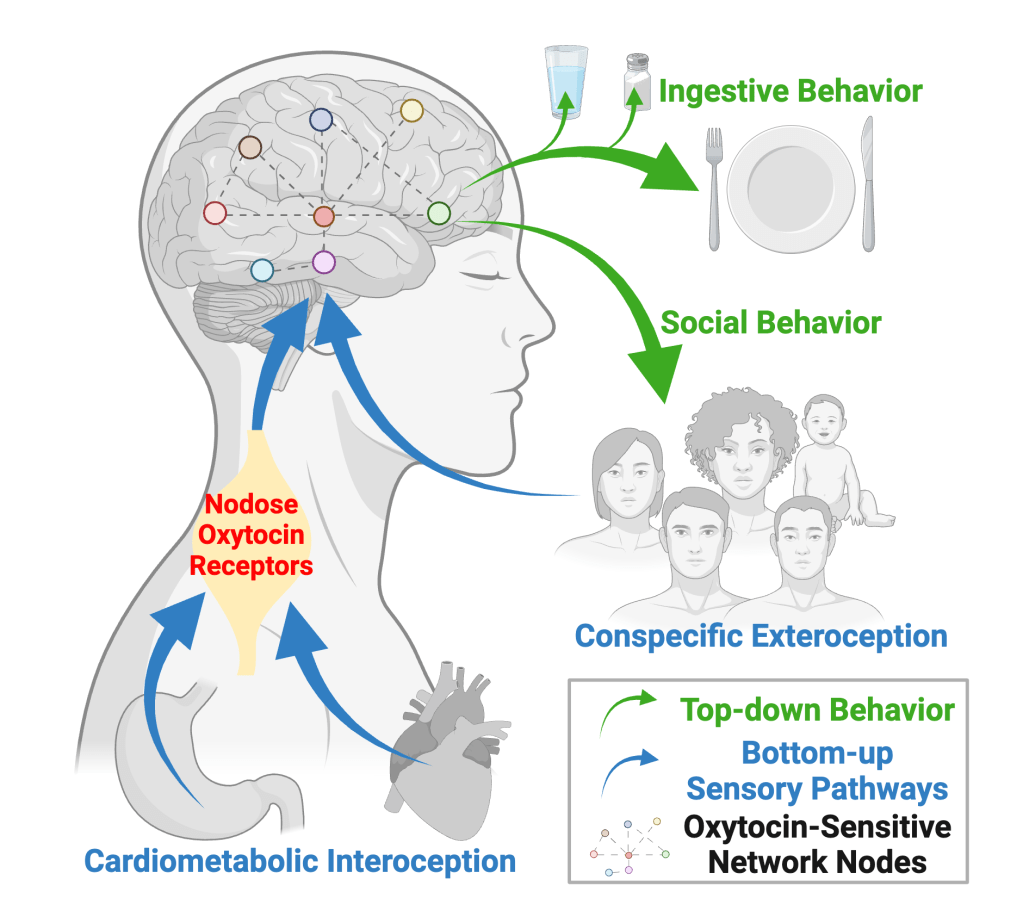
Vagal sensory neurons that express the oxytocin receptor innervate the gut and the heart. These neurons are associated with communicating information about stretch from the vasculature or GI tract to the brain.11
We’re testing the hypothesis that distinct populations of oxytocin receptor-expressing neurons within the NG form separate neural circuits that relay information about blood pressure or gastric distension, and that simultaneous activation of these separate populations triggers a state of torpor. We hypothesize that this mechanism serves to protect animals when external stressors prevent meeting internal needs.
Neural Circuits Controlling the Perception of Stress
In collaboration with Eric Krause, PhD and Annette de Kloet, PhD at Georgia State University
While there is consensus that stress negatively impacts cardiovascular health, the mechanism
underling this relationship is poorly understood.12
The nervous system orchestrates behavioral and physiological responses associated with stress and we are exploring the neural circuits controlling the perception of stress and whether these can be altered to prevent the pathophysiology that promotes cardiovascular disease.


References
- de Lartigue, G. Role of the vagus nerve in the development and treatment of diet‐induced obesity. J Physiol. 2016;594(20): 5791–5815.
- Lee, S.J., et al. Blunted Vagal Cocaine- and Amphetamine-Regulated Transcript Promotes Hyperphagia and Weight Gain. Cell Rep. 2020;30(6): 2028–2039.e4.
- Martin, A.M., et al. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front Physiol. 2019;10.
- Belkaid Y, et al. Role of the Microbiota in Immunity and Inflammation. Cell 2014;157(1):121-141.
- Ley, R.E., et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 2005;102:1070-11075
- Turnbaugh, P.J., et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008;17;3(4):213-23
- Kim, J.S., et al. The gut-brain axis mediates bacterial driven modulation of reward signaling. Mol Metab. 2023;75:101764
- Centers for Disease Control. Facts About Hypertension. Available at: cdc.gov/bloodpressure/facts.htm. Last updated July 6, 2023
- Zubcevic, J., et al. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ Res. 2019;125:104-116.
- Ansari, P., et al. Diabetic Retinopathy: An Overview on Mechanisms, Pathophysiology and Pharmacotherapy. Diabetology 2022;3(1), 159-175
- Bai, L., et al. Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 2019;79(5):1129-1143
- Dar, T., et al. Psychosocial Stress and Cardiovascular Disease. Curr Treat Options Cardiovasc Med. 2019;21(5):23